Stochastic Non-Equilibrium Thermodyamics
|
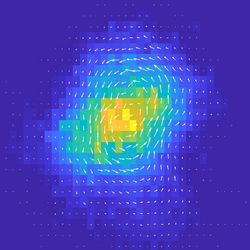
We are interested in measuring a distance from equilibrium, and relating this to changes in material phase, and to the function of biological systems. Ben Machta (Yale Physics) develops novel frameworks for estimating the irreversibility of non-equilibrium systems which have proven powerful for understanding transitions in non-linear systems. Thus, we apply these frameworks to simplied and controllable ‘active’ experimental systems that exhibit stochastic dynamics. We also study these systems in simulations of reaction-diffusion dynamics. Publications: [Nat Comms, 2018][Adv Func Mat, 2020],[Nat Comms, 2021] People: Daniel S. Seara, Vikrant Yadav |
 |
Experimental Biological De Novo Assembly
|
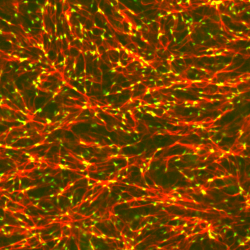
The actin cortex, the mechanical machinery within the cell, is a disordered random polymer network attached to the inside of the cell membrane. Myosin II is a molecular motor which grips the network and contracts it, generating mechanical forces. How the motors contract the network, and how attachment to a fluid membrane modulates this contractility is unknown, as we do not have precise control over these components in cells, and cannot visualize them with high fidelity. To address this problem, we ‘mimic’ the actin cortex outside of the cell by coupling isolated F-actin (right, red), myosin II (right, green) and its attachment to the membrane (not seen). We induce contraction and study the behavior of individual F-actin to explain the mechanism behind cell contractility. Publication: [Adv Func Mat, 2021][Nat Rev Mol Biol, 2015],[Mol Biol Cell, 2014],[PNAS, 2013],[PNAS, 2012] (right) Remodeling of an F-actin network (red) by myosin II (green) |
 |
Mechanics of ‘Active’ Interfaces
|
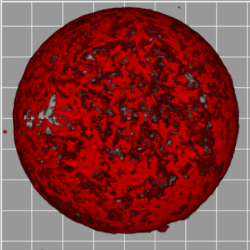
One of the ways cells change their shape is when they attach to an adhesive surface, like the extracellular matrix (ECM). Cells ‘spread’ over the ECM, creating a large, flat interface. Dynamically, the early stages of cell spreading are very similar across many cell types. It is presumed that the actin cortex, the structural machinery in the cell, is responsible for governing the dynamics of this shape change, although in real cells, there are too many components to to quantiatively dissect what its specific role is. So we create simplified, artificial versions of the cell, with only model membranes and purified actin. In this work, I probed the contribution of the synthetic cortex to the mechanical properties of the membrane through comparing the dynamics of adhesion and spreading of vesicles that carry this actin cortex to vesicles that do not. We find that the actin (red, right) contributes an enhanced viscosity to the membrane, and this viscosity dampens the dynamics of cell spreading(far right). Publication: [Nat Phys, 2019],[Nat Phys, 2014],[BJ, 2011] (right) F-actin network within a liposome. |
 |
Active Wetting of Living Cells & Tissues
|
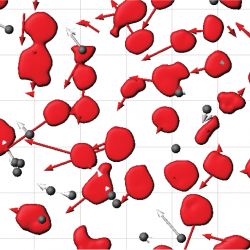
The collective motion that is seen in the movement of epithelial cells is influenced by the balance between the mechanical forces cells exerted against the extracellular matrix (ECM) to propel themselves, as well as the mechanical forces that are generated between each other at cell-cell contacts. In this work, we show that when the ECM is not only compliant, but is viscous, that the tension that exists between cells enhances the correlation in motion. In this case, motion is not caused by single cell traction against the ECM, but through contraction of the cell sheet as a whole, which induces a flow in the ECM itself Publication: [Nat Phys, 2019],[BJ, 2011],[PLoS One, 2011] (right) Shape Identification and Dynamic Position Tracking of Cell Nuclei in a Migrating Epithelial Sheet |
 |